- Image via Wikipedia
The Perceval S Sutureless biological valve is indicated for the rapid and minimally invasive surgical replacement of the aortic valve in patients suffering from aortic stenosis. Aortic stenosis is the most common acquired valvular heart disease in the western world and its occurrence increases with age. The prevalence of severe symptomatic aortic stenosis is typically reported around 3% in patients 75 years of age (this percentage also rises steeply with increasing age). The prognosis is poor for patients with severe symptomatic aortic stenosis and if after the onset of symptoms the patient remains untreated, the average survival is short – often only 2 to 3 years.
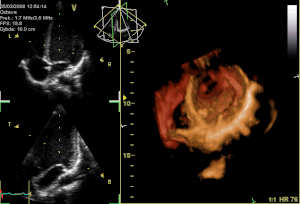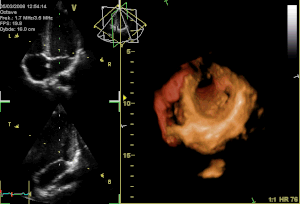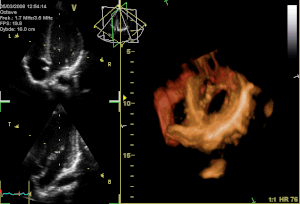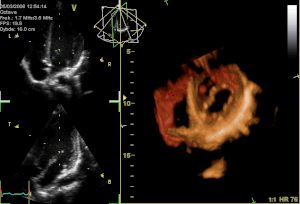
Perceval S is a surgical aortic valve with a unique self-anchoring frame that enables the surgeon to replace the diseased valve without suturing it into place, therefore representing an evolution beyond traditional bioprostheses. The valve’s functional component is made of bovine pericardium and is mounted on a super-elastic alloy frame. This state-of-the-art system builds on a clinically proven stentless aortic pericardial valve implanted in over 10,000 patients with proven durability and optimal hemodynamics. Clinical results in the first 180 patients implanted with Perceval S show a significant reduction in surgical procedural time for both isolated and complex aortic valve replacement with aortic cross-clamp times typically reduced by at least 50%. Perceval S leverages the reliability of gold-standard cardiac surgical results. The hemodynamic performance was outstanding with low pressure gradients and large effective orifice areas at 1 and 2 years follow-up. To date, 25 cardiac surgery centers throughout Europe have implanted the Perceval S valve in over 500 total patients as part of the pre-commercialisation clinical studies.
“We are delighted to receive the CE Mark approval for this revolutionary technology. We are dedicated to providing cardiac surgeons worldwide with high performing innovative therapeutic solutions that will enhance their practice and advance the treatment of cardiovascular diseases. This further strengthens our leadership in heart valve replacement technologies and gives us a competitive advantage in this advanced clinical segment,” said Davide Bianchi, President Heart Valves Business Unit, Sorin Group.
About Sorin Group
Sorin Group (www.sorin.com), is a global medical device company and a leader in the treatment of cardiovascular diseases. The Company develops, manufactures and markets medical technologies for cardiac surgery and for the treatment of cardiac rhythm disorders. With 3,600 employees worldwide, Sorin Group focuses on three major therapeutic areas that include: cardiopulmonary bypass (extracorporeal circulation and autotransfusion systems), cardiac rhythm management, and heart valve repair and replacement. Every year, over one million patients are treated with Sorin Group devices in more than 80 countries.
